Pelvic Health Company Raises $20M in Billion-Dollar Femtech Market
SexForEveryBody.com is supported by our readers. We may earn a commission if you buy through links on our site. Learn more.

Materna Medical, a pelvic health company that aims to change the standard of care during pregnancy and childbirth, announced it secured a $20 million investment from private equity firm InnovaHealth in August 2022.
According to CEO Tracy MacNeal, the OGBYN platform targets a $6 billion market that is underserved and ripe for disruption. That motivated her to become President and CEO in 2019, nine years after Materna Medical was founded.

“I rarely see a startup with this market size with so little competition and so much risk removed,” MacNeal said while presenting at the LSI USA ‘22 Emerging Medtech Summit in July.
Prior to joining the femtech company, MacNeal spent more than 15 years working at the C-suite level of medical device companies and launched more than ten products.
Materna Medical’s two medical devices aim to protect and restore pelvic floor health. The vaginal dilator Milli received FDA clearance to help treat the symptoms of vaginismus, a condition that causes involuntary contracting of vagina muscles during penetration.
The femtech company’s second product is designed to prevent pelvic floor injuries during childbirth. Called Materna Prep, the device is undergoing clinical trials on first-time mothers to test its safety and effectiveness.
Growing OBGYN rehab market
The thriving market for women’s pelvic health, and its projected growth, are fueled by various factors.
Market intelligence company Fior Markets predicts that the global women’s health rehabilitation market will reach US$6 billion by 2028. That represents an average growth rate of 6% a year, counting up from US$3.69 billion in 2020.
A growing elderly female population, an increase in chronic illness, and the rise of the medical device and assistive technology markets are all driving forces.
In 2020, urinary incontinence represented the largest segment of this market at 18%.
Pelvic floor muscle dysfunction, in particular, is linked to common musculoskeletal issues that occur during pregnancy, childbirth, post-partum, and menopause.
Related Read: Exploring Cliovana: Soundwave Therapy for Vaginal Health
Disrupting pelvic floor dysfunction
One reason the market is so large, MacNeal points out, is that childbirth is the number one reason for hospitalization around the world.
Studies also show that 90% of vaginal deliveries cause tears in the perineum, the tissue between the vagina and the anus.
What is less understood, says MacNeal, is that 50% of women will experience prolapse or incontinence by the age of 55. These conditions also represent the top two reasons why older women are admitted into nursing homes.
“That can happen just from gravity but we are nine times more likely to have those symptoms if we’ve had a vaginal delivery, and yet the standard of care in this aspect of childbirth is the same as it’s been since the dawn of time: you just hope for the best,” she says.
A preventative approach to pelvic health
The first of its kind, Materna Prep aims to make vaginal deliveries safer by reducing the risk of pelvic floor muscle injuries. It was created at Stanford’s Byers Center for Biodesign and is undergoing its second year of clinical trials.
The premise behind Materna Prep is that slow stretching of the pelvic floor will prevent muscle tears.
Pelvic floor muscles are visco-elastic, explains MacNeal. If they are suddenly stretched they remain rigid and will tear. On the other hand, if they are stretched slowly they will remain elastic and less prone to injury.
To prepare for vaginal childbirth, the device enters into the birth canal when the cervix is 6cm dilated. It can be given with an epidural.
Related Read: Vulvodynia: Symptoms, Causes, and Treatment Options for Chronic Vulvar Pain
Upon entry, its diameter is 3.4cm, just a bit smaller than a US quarter. It then gradually expands to 8cm, just under the average baby’s head size of 10cm. After the pelvic floor muscles can stay stretched for up to three hours and allow for vaginal delivery.
According to MacNeal, the pelvic health device has the potential to greatly reduce hospital costs by lowering C-sections, birth complications, readmission rates, and the risks of post-partum hemorrhage and blood infections in newborns.
The latest $20 million investment will be put toward getting Materna Prep into FDA Clearance, which is projected to happen in late 2023.
The vaginal dilator Milli
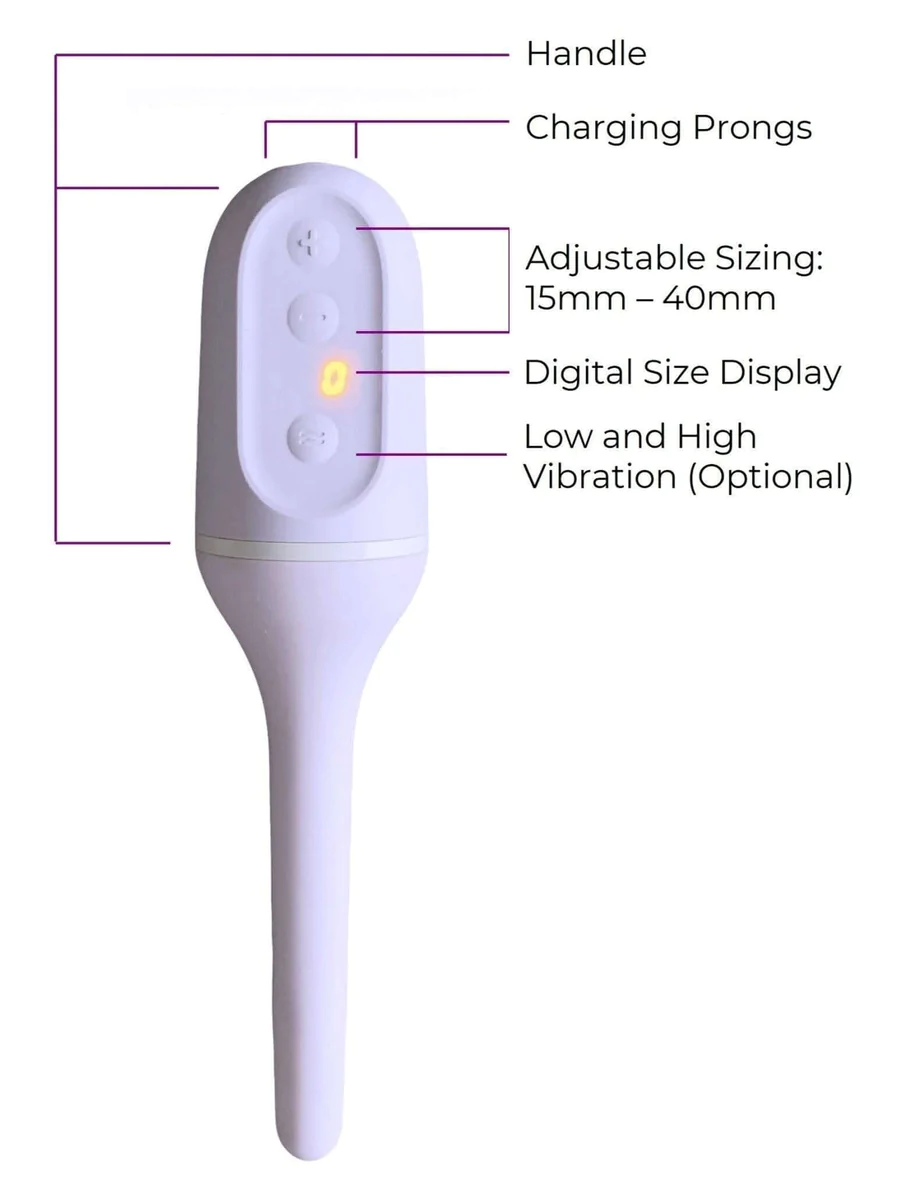
Milli is a vaginal dilator that was launched in 2019 to treat chronically tight pelvic floors. The medical device for pelvic health gained FDA Clearance this year and made $300,000 in revenue in 2020.
On a video call, MacNeal told SexForEveryBody.com that the vaginal dilator is meant to help women who experience painful sex or are unable to have penetrative sex—a condition often referred to as vaginismus.
Related Read: MysteryVibe FSA Eligible Vibrators
Vaginismus is considered one of the most common types of sexual dysfunction experienced by women, with a prevalence rate estimated between 5% and 17% in a clinical setting.
The vaginal dilator is made of silicone and expands from 15mm to 40mm. It also offers low and high-vibration settings, which can help reset nerve endings.
Like Materna Prep, Milli was also created in collaboration with Stanford’s Byers Center for Biodesign
The pelvic health device can be used alone or with a partner. Women with partners are encouraged to talk to them about what they may need to feel supported.

Jenna Owsianik is a Canadian journalist and sex tech industry expert. She is the Founder and Editor-in-Chief of Sex For Every Body®.
Her expertise covers state-of-the-art sex technologies and the major fields driving innovations in intimacy: robotics, virtual reality, remote sex (teledildonics), haptics, immersive adult entertainment, human augmentation, virtual sex, and sexual health.
A trained journalist with a Masters of Journalism from The University of British Columbia, Jenna’s reporting has appeared on Futurism.com, Al Jazeera English, CTV British Columbia online, CBS Sunday Morning, CBS 60 Minutes, Global News, and CKNW Radio in Canada and the United States.









